Accurate disease diagnosis depends on identifying symptoms and indicators, which can manifest differently in women and men. Medication and treatment plans are just as important as the initial diagnosis and have varying effects based on sex. However, these differences have been insufficiently documented, according to the National Institutes of Health.
Historically, disease symptoms present in men have been thoroughly studied, according to the NIH. Men have also made up the majority of participants in clinical trials. In contrast, women have been excluded from medical studies of symptoms and clinical trials.
Undocumented symptom differences generally occur due to males being considered the “standard” body in medicine and the unfounded assumption that females will present the same symptoms — an assumption that has not been challenged for centuries, according to the CommonWealthFund. The gap in knowledge is largely attributed to misogyny and bias, according to Medidata.
One approach assumes medical treatments have identical effects in both men and women. The other excludes women from clinical trials to prevent hormonal differences between the sexes from confounding the data and affecting the overall treatment effectiveness. However, it is important to determine whether the effectiveness of a medical treatment varies between sexes. Even after women were included, the data was still not analyzed by sex to assess the distinct effects of treatments on men and women. Men continued to dominate clinical trial participation, so even when women were included, data remained skewed, primarily reflecting male results.
Excluding women for reasons that are rooted in bias leaves them at a disadvantage. Different symptoms lead to a delayed diagnosis, misdiagnosis or even symptoms being dismissed as psychological, according to the NIH. This allows diseases to progress, worsening outcomes. Inadequate treatment testing complicates the understanding of real-world effects, outside clinical testing. While progress is being made, the issue remains prominent, according to FHS science teacher Julia Coe.
“I think it’s really important that we consider how to best and most ethically recruit and enroll diverse sets of people in these clinical trials [during] a human trial,” Coe said.
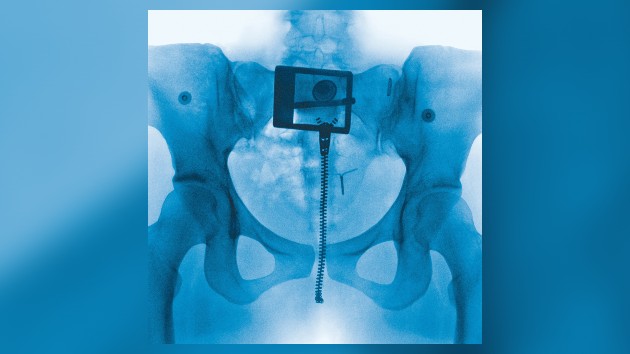
Between 2011 and 2019, Coe worked as a clinical research coordinator recruiting patients for clinical trials testing surgical devices in spinal surgeries. They recruited people from a clinic for something affecting both sexes, so patients in trials included both sexes. However, it becomes harder to recruit a diverse set of people in trials testing treatment for a disease that affects men and women differently, determining its final demographics. This makes it harder to close the medical research gap between males and females, even when it is attempted.
Being aware of and informed about the medical research gap between sexes is the first step to narrowing it. With action, the gap may be bridged.
“The human body is so wonderfully and mysteriously complex,” Coe said. “We know a lot about it now that we didn’t before, but there’s still a lot we don’t know.”